Prof. Dr. Saim Özkar
Research GroupMetal nanoclusters
exhibit unique properties which differ from their bulk
materials, owing to the quantum size effects. However,
nanoclusters tend to be fairly unstable in solution and thus
special precautions have to be taken to avoid their aggregation
or precipitation during the preparation of such nanoclusters in
solution . In order to obtain stable nanoclusters dispersed in
solution, a stabilizing agent is usually added into the reaction
system. In the literature of colloidal stability and in
Derjaguin-Landau-Verway-Overbeek (DLVO) theory, colloidal
stabilization is well established to involve both: (i)
electrostatic stabilization by the surface adsorbed anions such
as chloride or citrate ions and (ii) steric stabilization by the
presence of polymers such as the often used
poly(vinylpyrrolidone). The use of polymeric matrix as
stabilizer improves some properties of the nanoclusters such as
the solubility, thermal stability and catalytic activity.
A variety of preparative
methods is available for obtaining polymer-stabilized metal
nanoclusters. The most widely used synthetic method involves
reduction of the metal ion in solution to the colloidal metal in
zerovalent state within the polymer medium, followed by
coalescence of the polymer onto the nanoclusters formed.
In our group, we have
developed many water soluble polymer stabilized transition
metal(0) nanoclusters by using (i) the reduction of metal
precursors in the presence of water soluble polymers by sodium
borohydride in methonol after 1 h reflux (Figure 1a, 1b, Figure
1c and Figure 1d (ii) in-situ reduction of metal
precursors in the presence of water soluble polymers in the
hydrolysis of ammonia borane (Figure 2a, 2b, 2c, 2d). Then, we
used water soluble transition metal(0) nanoclusters as catalyst
in hydrogen generation from the hydrolysis of sodium
borohydrides and ammonia borane.
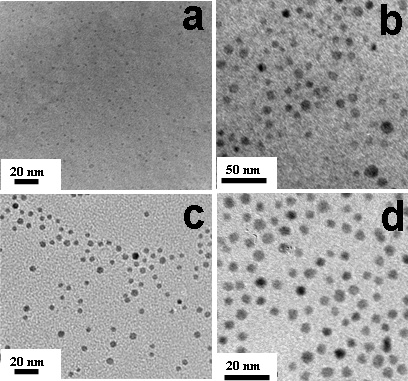
TEM images of
poly(N-vinyl-2-pyrrolidone) stabilized
(a) Ni(0)
Nanoclusters (b) Co(0) Nanoclusters (c) Ru(0)
Nanoclusters (d) Pd(0) Nanoclusters