Prof. Dr. Saim Özkar
Research GroupThe activity of catalyst is directly related to its surface area, for this reason the use of transition metal nanoclusters is a promising way to increase the catalytic activity with respect to their bulk counterparts. However, in their catalytic application one of the most important problems is the aggregation of nanoclusters into clumps and ultimately to the bulk metal, despite of using the best stabilizers, which leads to decrease in catalytic activity and lifetime. The use of nanoclusters catalysts in systems with confined void spaces such as inside mesoporous and microporous solids appears to be an efficient way of preventing aggregation. In this regard, Zeolite-Y and ZK-4 are considered as a suitable host providing highly ordered large cavities (supercages). At this point the aim of our group project is to prepare zerovalent Ru, Rh, Ir, Os, and Au nanoclusters within the cages of zeolite-Y and ZK-4 and check their catalytic activities in various reactions depending on the nature of activity of metals (oxidation of alcohols, dehydrogenation of amine-borane adducts, hydrogenation of olefins and arenes, and the hyrolysis of sodium borohydride). To characterize the structure of the intrazeolite transition metal nanoclusters XRD, XPS, ICP-MS, SEM, HRTEM, Raman, Mid and Far-IR, N2 Adsorption, EXAFS, XANES and NMR, UV-Vis and Diffuse Reflectance spectroscopy were used.
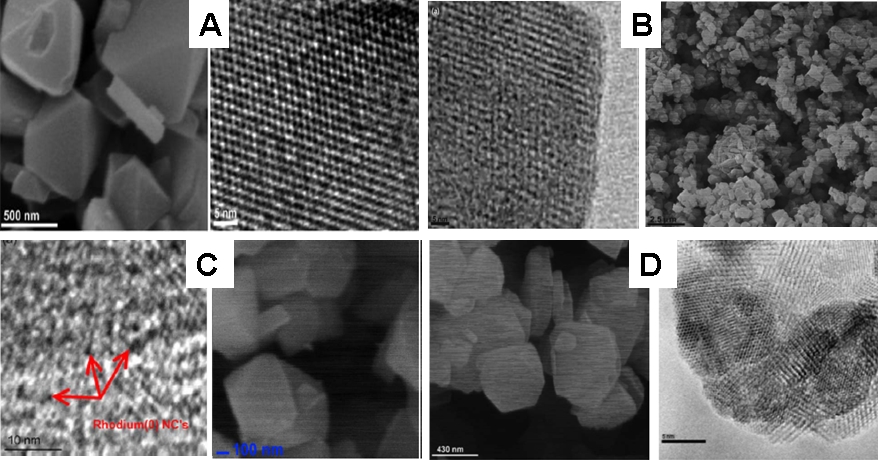
TEM and SEM images of intrazeolite (A) Ru(0) nanoclusters (B) Au(0) nanoclusters (C) Rh(0) nanoclusters (D) Co(0) nanoclusters